Ever since Dr. John Snow established in 1854 that water could be a mode of transmission for deadly diseases such as cholera, water disinfection is a basic requirement in all drinking water treatment process. Chlorine, the first disinfection chemical discovered in 1774 by the chemist Karl Scheele (Centers for Disease Control and Prevention, 2016), was used for this purpose for the first time in large scale in 1908, when Dr. John Leal with the help of George W. Fuller started the first chlorination facility in the United States for drinking water disinfection (McGuire, 2013). The use of chlorine reduced significantly waterborne diseases all over the western world and also, unfortunately in a much slower way, in developing or poor countries. The 2017 report from United Nations Children’s Fund (UNICEF) and World Health Organization (WHO) - Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselinesconcluded that 2.1 billion people worldwide still lack access to safe readily available water.
Despite treated safe water being crucial to avoid waterborne diseases it is known, since the mid-1970s, that the process of disinfection produces by-products (DBPs) that can be a hazard to human health, being chloroform and other trihalomethanes the compounds that created the first concerns in the scientific community (Karlin, 1999).
Since then, other chemicals and techniques for water disinfection have been used in order to kill pathogens in drinking water. For instance, chlorine dioxide, ozone, chloramine and in some cases combinations of them are being used in modern water treatment systems worldwide and each one of them, depending on a number of factors, may be derived into DBPs.
The most used association between DBPs and adverse health outcomes is the consume of chlorinated water and bladder cancer (although children born small for gestational age and miscarriages have also been reported). In a study about water disinfection by-products and bladder cancer, where a pool and a two-stage random-effect metaanalysis of three European case-control studies was conducted, bladder cancer was 47 % more prevalent among those consuming water with THM4 > 50 μg/L compared to those consuming water with THM4 < 5 μg/L (Mitch, 2018).
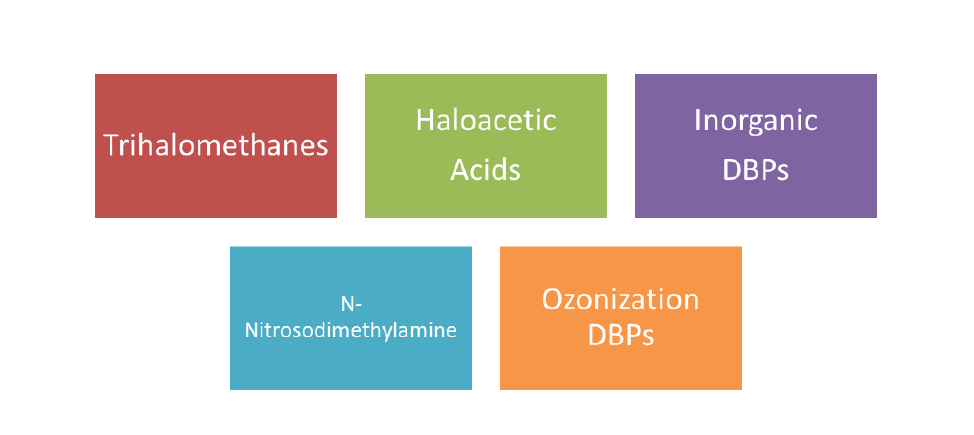
DPBs can be categorized into the following groups:
What affects DBPs formation?
DBPs are formed by the reaction of natural organic matter (NOM) or inorganic substances in water (e.g. chloride, bromide) with the disinfectant chemical. This reaction may be affected by pH, temperature, concentration of the precursor or concentration of the disinfectant. The table below summarizes the effects of some of these parameters in the formation of DBPs.
INSERT IMAGE HERE
How to prevent/control the formation of DBPs?
Different methods to control DBPs have been developed. These methods can be preventive or corrective. Preventive methods look for reducing the probability to form DBPs (e.g. reduction of NOM, use of alternative disinfectants, enhanced coagulation, etc.). Corrective methods are oriented to reduce DBPs that are already formed (e.g. granular activated carbon adsorption).
Preventive methods
INSERT IMAGE HERE
- Enhanced coagulation: It is defined as an optimized coagulation process for removing DBP precursors, or natural organic matter (NOM). NOM is measured as total organic carbon (TOC) or dissolver organic carbon (DOC). In general, enhanced coagulation is practiced at a higher coagulant dose and a lower pH. Considering that the process is done at a low pH, we need to consider the alkalinity of water, as it is more difficult to achieve a low pH in high alkalinity source waters. Coagulants that can be used are alum, ferric chloride, ferric sulfate and ferrous sulfate. The dose of coagulants must be established by jar tests and may vary from 9 to 60 mg/L, depending on the TOC of the water source. The conditions that affects TOC removal include alkalinity, pH, turbidity, TOC concentration, origin of NOM, temperature, coagulant dosage and type, peroxidation, mixing, among others, but the operations that greatly affect TOC removal are coagulant type and pH pre-adjustment.
- Carbon adsorption: Carbon adsorption can be used to remove DBP precursors and DBPs. Both granular activated carbon (GAC) and powdered activated carbon (PAC) are used for these applications. Carbon filters must be designed in order to achieve an EBCT (empty bed contact time) of at least 10 minutes, and in some cases EBCT can be as high as 30 minutes. A pilot study is recommended to evaluate carbon adsorption performance.
- Changing chlorination point: Moving back the chlorination point or eliminating pre-chlorination points are two effective ways to control DBP levels in finished waters. Alternatively, a peroxidation step with alternative disinfectants or oxidizers (e.g. potassium permanganate or chlorine dioxide) can be used.
- Use of membrane technologies: Nanofiltration is an extremely effective method for reduction of DBP precursors and, if a proper pretreatment is applied, ultrafiltration and microfiltration can be effective too. Traditionally, membrane technologies are more used in groundwater treatment systems, but by using a correct pre- and posttreatment, it can be used in surface water
- Use of alternative disinfectants: Alternative disinfectants include chloramines, ozone, dioxide chloride and UV.
- Chloramine is a much weaker disinfectant than free chlorine as it requires more contact time for an adequate disinfection, and lasts longer in distribution systems. However, chloramine also produces DBPs: cyanogen chloride (or cyanogen bromide in case of high bromide concentration), THMs and HAAs. The formation of these will depend on the chorine to ammonia ratio.
- Ozone is a stronger disinfectant than free chlorine and it is very effective against microorganisms like Giardiaand Cryptosporidium. It is highly reactive, so it does not provide a long lasting residual in distribution systems. It reacts with bromide to produce bromate and hypobromous acid that reacts with NOM to produce brominated DBPs. In this case, pH and temperature must be controlled to reduce DBPs formation.
- Chlorine dioxide is a stronger bactericide and viricide with a longlasting residual in distribution systems. Chlorine dioxide may produce chlorite as a DBP and with the effect of ozone or chlorine it may produce chlorate.
- UV radiation provides a physical method for disinfection and it is very effective against bacteria and viruses. UV does not leave a disinfectant residual so it is normally used with a secondary disinfectant like chlorine, chloramine or chlorine dioxide.
Corrective methods
Carbon adsorption: So far, carbon adsorption is the most common method to reduce DBPs in water once they are already formed. Granular activated carbon filtration has been used effectively to reduce HAAs and THMs in finished water. The treatment is more effective if biologically active carbon (BAC) is used because biological degradation of HAAs contributes to the reduction of DBPs.
Conclusion
In January’s article 6 steps were presented to manage water quality in the food industry. In step 1 – Define sources and purposes the importance of knowing the characteristics of the water used right from the source is highlighted. This is also essential when you are designing a water treatment process. One thing if for sure, it is very unlikely that it does not generate disinfection by-products. However, treatment conditions can be optimized to reduce their formation in order to provide water that can be consumed safely.
We, food safety professionals, should always embrace preventive and risk-based approaches to hazards, and the better you know in detail the quality of the source water, the better you can anticipate, monitor and manage the formation of DBPs.
Despite all the above and the growing and growing concern regarding the long term health effects of the consumption of water with DBPs, we must always have in mind (specially in countries where safe water is not readily available) that according to the WHO: Infectious diseases caused by pathogenic bacteria, viruses, protozoa, and helminths are the most common and widespread health risk associated with drinking-water.
Consequently, WHO states: Where local circumstances require that a choice must be made between meeting either microbiological guidelines or guidelines for disinfectants or disinfectant by-products, the microbiological quality must always take precedence, and where necessary, a chemical guideline value can be adopted corresponding to a higher level of risk. Efficient disinfection must never be compromised.
YOU CAN REACH THE AUTHORS AT:
Nuno F. Soares - foodsafetybooks@gmail.com
Aldo Estela -aldo.david@gmail.com
Disclaimer: The information contained on this article is based on research done in the last months and the authors personal experience and opinion. It is not intended to represent the view of any organization they work for or collaborate with. The author will not be held liable for the use or misuse of the information provided in the article
About the Author:
Nuno F. Soares, Ph.D., is an author, consultant, and trainer in food safety. He is a Food Engineer Specialist and senior member of the Portuguese Engineering Professional Association.
He has over 20 years of experience in food industry as quality and plant manager. His latest book is “ISO 22000:2018 Explained in 25 Diagrams.”