One of the primary reactions to the current Listeriosis outbreak has been to clean, clean, clean... and rightly so. But given the effort and expense it takes to clean, it is essential that cleaning is undertaken in the correct way to ensure the maximum benefit.
"Sanitation is the foundation for an effective
Listeria Control Program." FSIS
The correct method of cleaning
A review of industry guidelines highlights the need for cleaning and disinfection programmes that specifically address L. monocytogenes to consist of three actions:
- effective removal of soil;
- an effective rinse step and
- proper application of a sanitizing agent, which includes contact time, concentration and temperature.
Your cleaning and disinfection programme
An effecive cleaning and disinfection programme should detail the following:
- Written procedures for proper cleaning and sanitizing Food contact surfaces (FCS) and non-FCS which should include the frequency of cleaning, chemicals to use, instruction on how to perform the task and the steps to verify it is being done correctly.
- A detailed cleaning schedule that clearly defines what must be cleaned and when, addressing all parts of the facility including drains, chillers and their drip trays, ceilings and other difficult to clean places.
- A visual examination of all FCS should be done before the start of operations to ensure compliance with cleaning procedures and to take corrective action if necessary.
- Written procedures for food establishments should include the cleaning and sanitizing of maintenance tools.
- Method/s for verifying the effectiveness of its cleaning and sanitation program.
- The person in charge should be responsible for ensuring that employees are properly trained for the tasks assigned to them and that they fully understand how to perform the sanitation procedures.
This doesn’t sound like rocket science but there is in the challenge – ensuring the food facility is free of Listeria monocytogenes requires focus on the basic PRP’s. This is especially critical in foods that are conducive the growth of the organism.
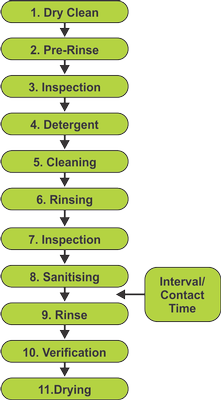
Recommended cleaning process for equipment
(FSAI)
The chemistry of cleaning
What to clean with is as important as what to clean. The choice of detergent depends on the type of soiling, on water hardness, the temperature of the method, the types of surfaces and safety. In general, alkaline detergents are used for the removal of organic soils, while acidic detergents are used on inorganic soils. Once cleaning has been completed, disinfection must be carried out using biocide appropriate to the type of surface material to be disinfected. A huge amount of research has been conducted into the comparative efficacy of disinfectants. The table below should be carefully considered when making your choice.
Table: The efficacy of sanitisers in removing L. monocytogenes contamination from poorly and properly cleaned surfaces or suspensions
|
(Reproduced from Hoelzer et al)
In the absence of protein residues (effective cleaning)
|
In the presence of protein residues (poor cleaning)
|
|
Sanitiser type
|
No. of
studies reviewed
|
No. of observations
|
Total
no. of replicates
|
Mean
reduction
(log cfu)
|
No. of
studies reviewed
|
No. of obser-
vations
|
Total
no. of replicates
|
Mean
reduction
(log cfu)
|
|
Acid-
anionic
|
3
|
39
|
78
|
7.1
|
1
|
4
|
32
|
5.3
|
|
Halogen
|
3
|
27
|
124
|
3.8
|
2
|
9
|
60
|
2.4
|
|
Hypochlorite
|
11
|
321
|
891
|
5.5
|
4
|
38
|
117
|
2.8
|
|
Peracetic
acid
|
6
|
177
|
484
|
4.6
|
2
|
24
|
52
|
3.8
|
|
Quaternary ammonium
|
5
|
59
|
262
|
6.1
|
2
|
8
|
56
|
5.3
|
Reminder from the Chilled Foods Association, UK
“A dirty surface cannot be disinfected effectively as the biocidal active cannot come into contact with bacteria trapped in and under soil. Extraneous organic material also dilutes and neutralises biocides before they are applied so surfaces must be cleaned. Neither large quantities of disinfectants nor high-pressure application can replace effective and thorough pre-disinfection cleaning.
Effective cleaning must therefore be carried out before applying a sanitiser to reduce the number of microorganisms.”
Selecting the right chemical
Given the current level of concern in industry regarding Listeria monocytogenes, there is possibility of opportunistic ventures. New suppliers and even new products can seem very attractive if you do have a problem with pathogen control. As with all new technology, it is prudent to ensure you do your homework in assessing the product and supplier. “Secret formulas” and “novel ingredients” should be interrogated to ensure these are supported by sound science. Using a certified cleaning chemical can greatly assist in providing confidence that the product is suitable for its application. Traditionally cleaning chemicals in South Africa have been certified to SANS 1853 and SANS 1828. Alternative schemes such as NSF White book registration can also be considered.

In addition to this voluntary certification, disinfectants are covered by legal requirements in most countries to confirm the stated efficacy. All disinfectants (for inanimate surfaces) sold in the Republic of South Africa are covered by VC 8054 - compulsory specification and regulated by the National Regulator for Compulsory Specifications, under the DTI. In addition to the requirements in this specification, the requirements promulgated under the Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act, 1947 (Act No. 36 of 1947), the Medicines and Related Substances Act, 1965 (Act No. 101 of 1965), and the Foodstuffs, Cosmetics and Disinfectants Act, 1972 (Act No. 54 of 1972) also apply. Biocides for use in water treatment are excluded.
If a disinfectant complies with the requirements of this compulsory specification, it can be considered to be bactericidal, fungicidal, sporicidal or virucidal, but it should not necessarily be inferred that it is suitable for a defined purpose.
"Sanitation actions should be escalated if repeated
positives are found, indicating Listeria trends." FSIS
Time for a cleaning review
Whether or not you have had a listeria problem, this outbreak should trigger a review of your HACCP plan. Remember Codex HACCP principles recommend that a review should take place if there is new epidemiological information such as emergence of a new food borne pathogen (e.g. bacteria that can cause illness) with public health significance or other health issue.
Although this is not a new pathogen, this outbreak is new for all of us and should prompt us to check all the controls we rely on – rather than assume.
References